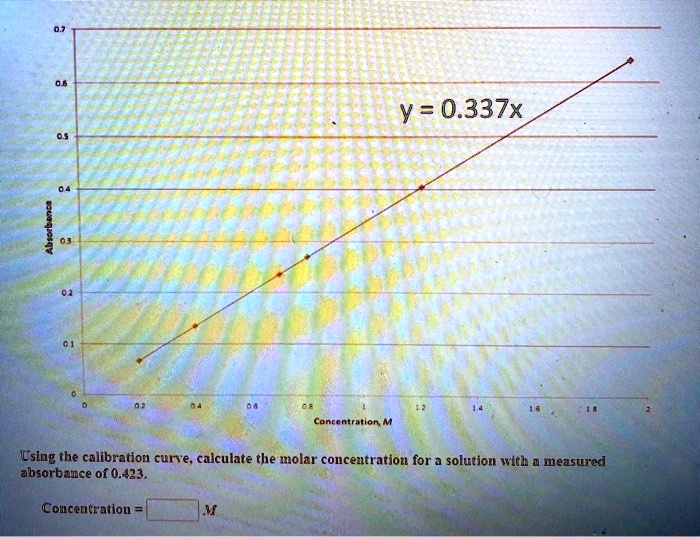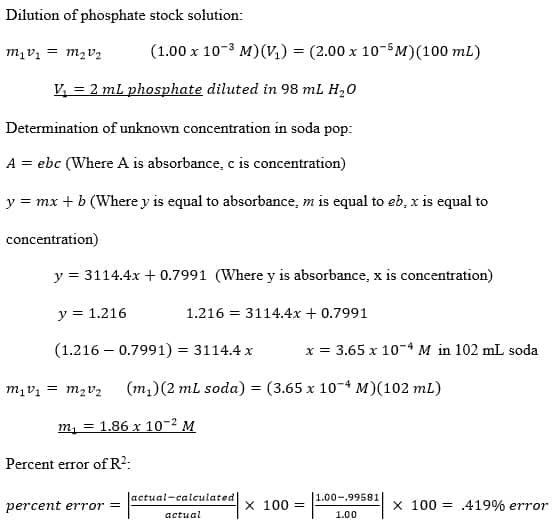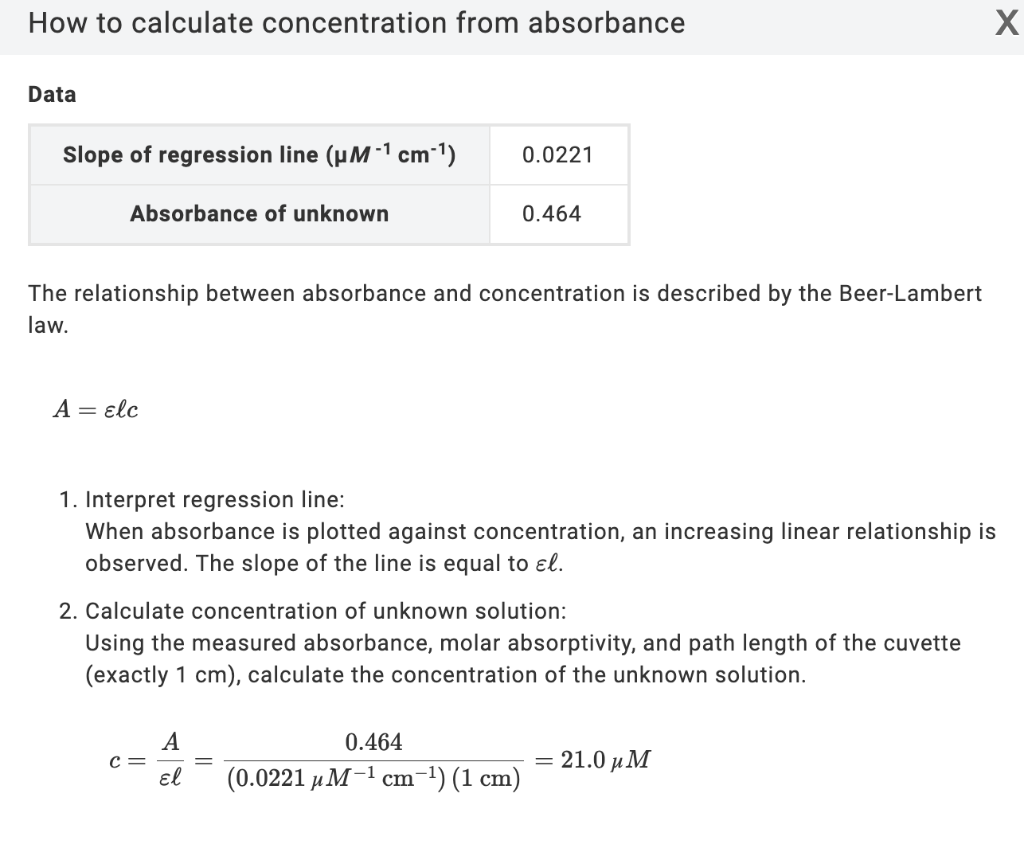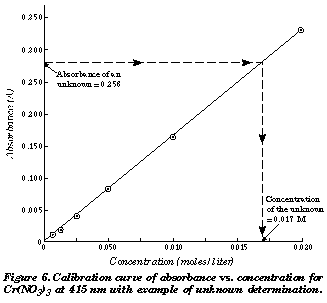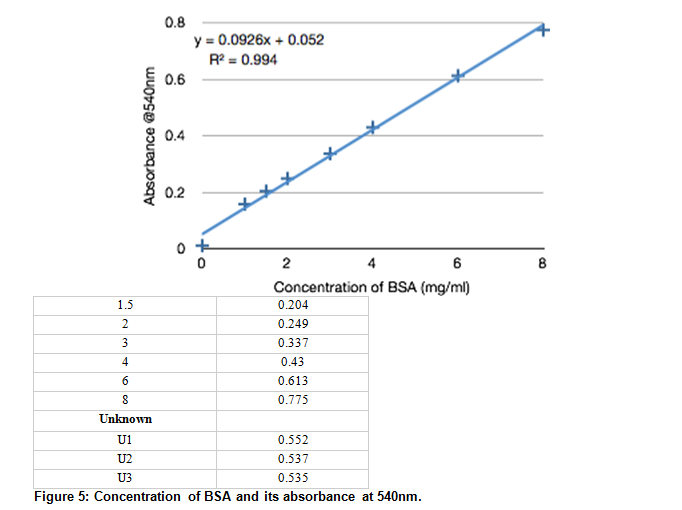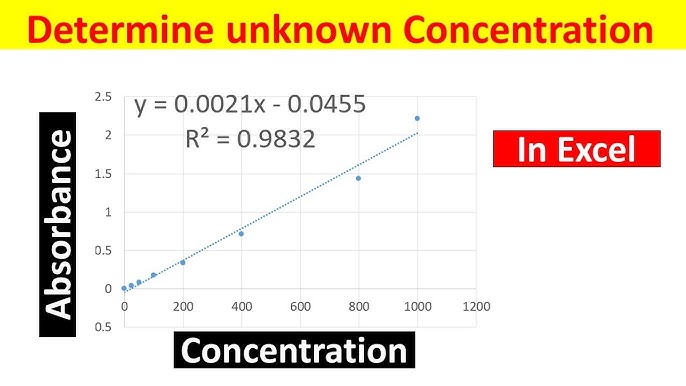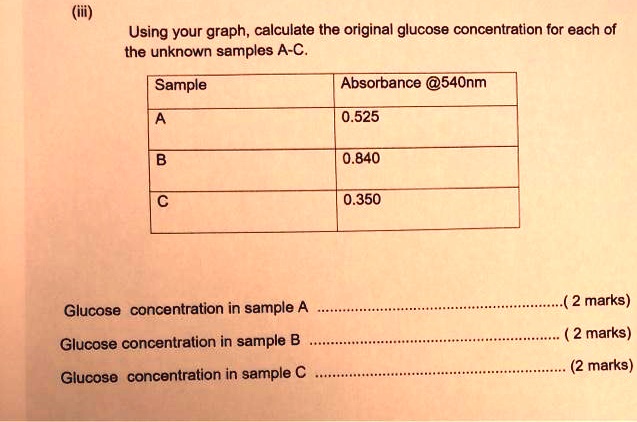
SOLVED: Using your graph, calculate the original glucose concentration for each of the unknown samples A-C. Sample Absorbance @540nm 0.525 0.840 0.350 Glucose concentration in sample A Glucose concentration in sample B
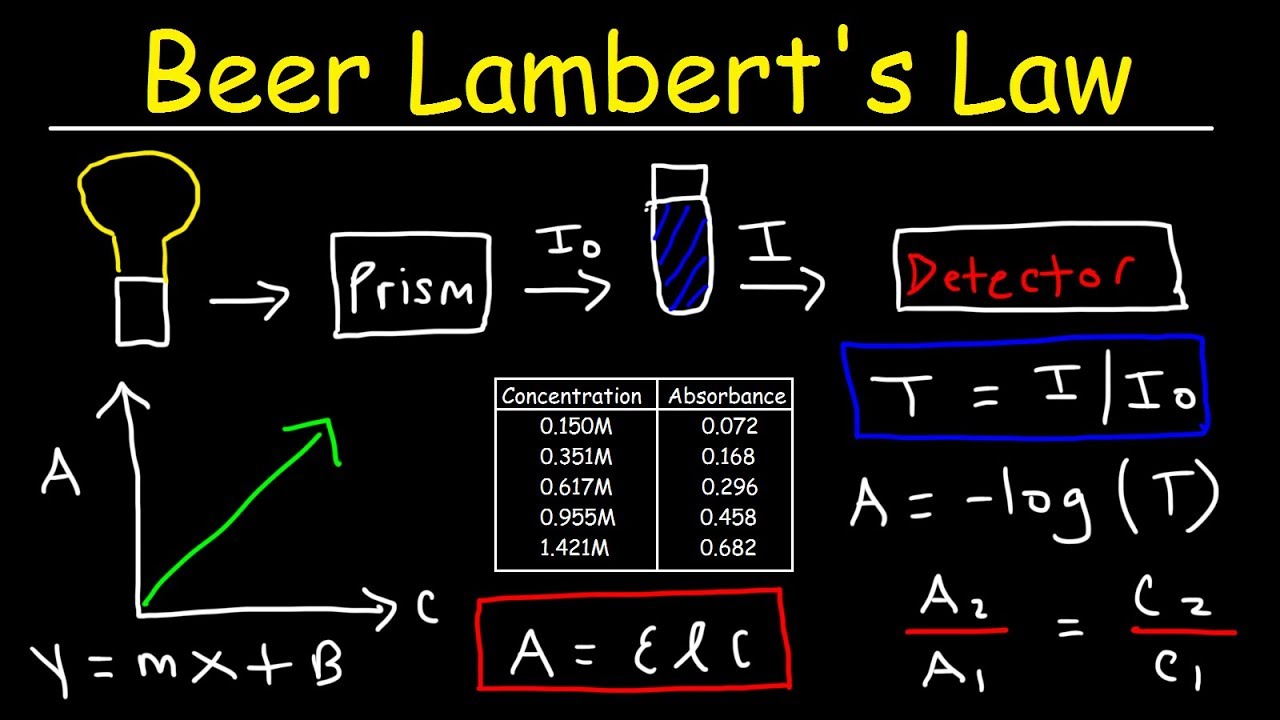
Beer Lambert's Law, Absorbance & Transmittance - Spectrophotometry, Basic Introduction - Chemistry - YouTube
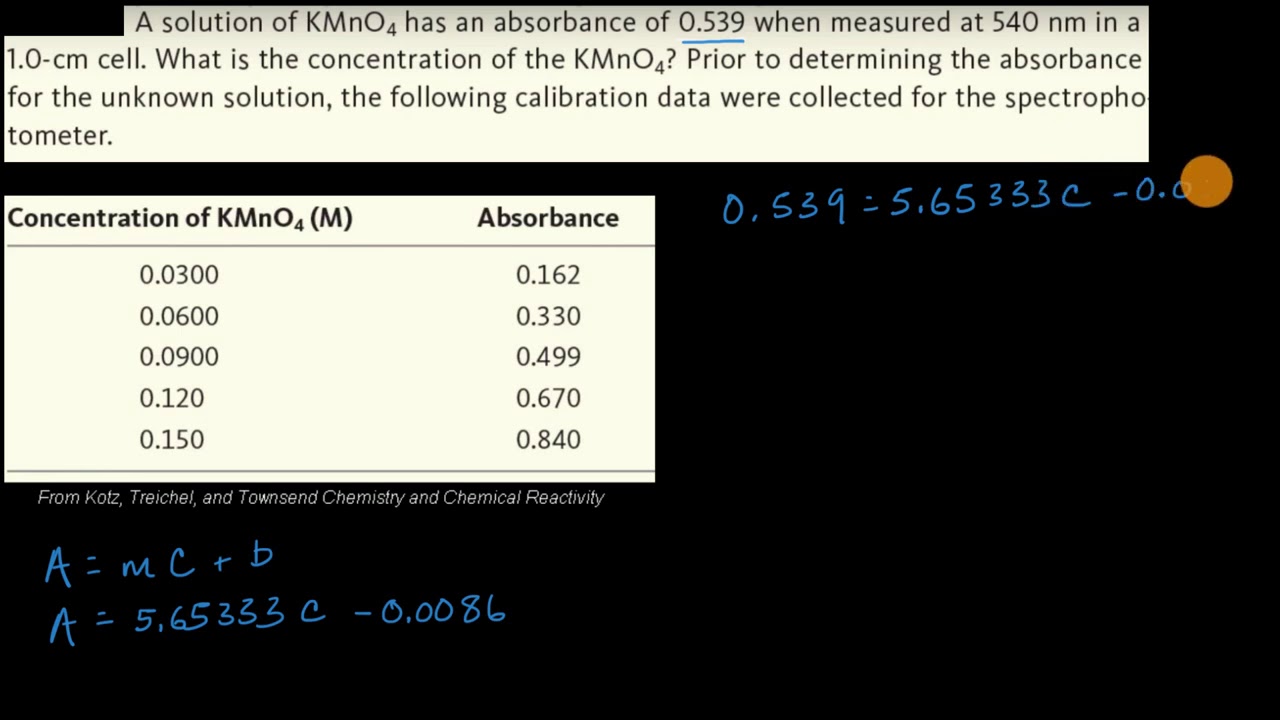
Worked example: Calculating concentration using the Beer–Lambert law | AP Chemistry | Khan Academy - YouTube
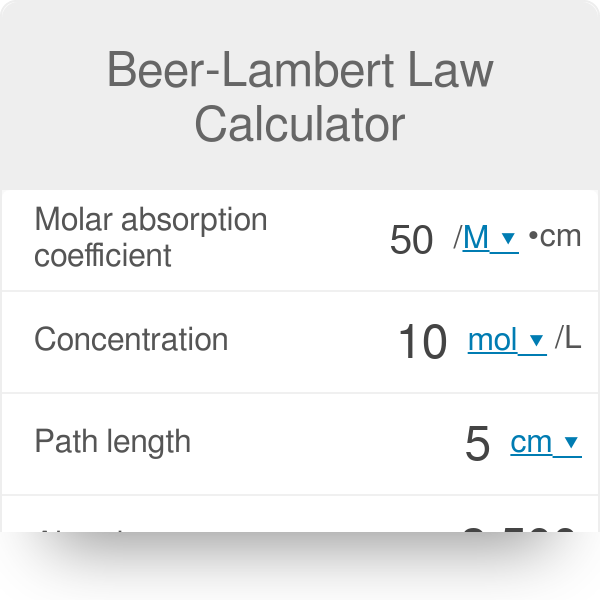
What is the absorbance of a 0.0024 M solution with a /molar absorptivity of 313 M- cm in a cell with a 2.00 cm path length? - Quora

An example of total iron concentration standard curve: absorbance at... | Download Scientific Diagram

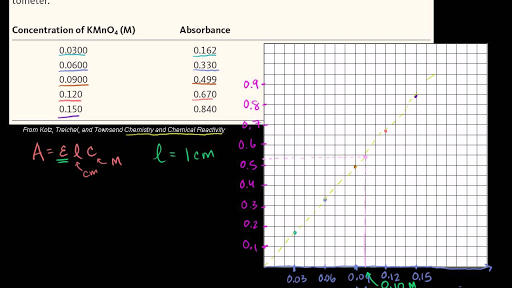
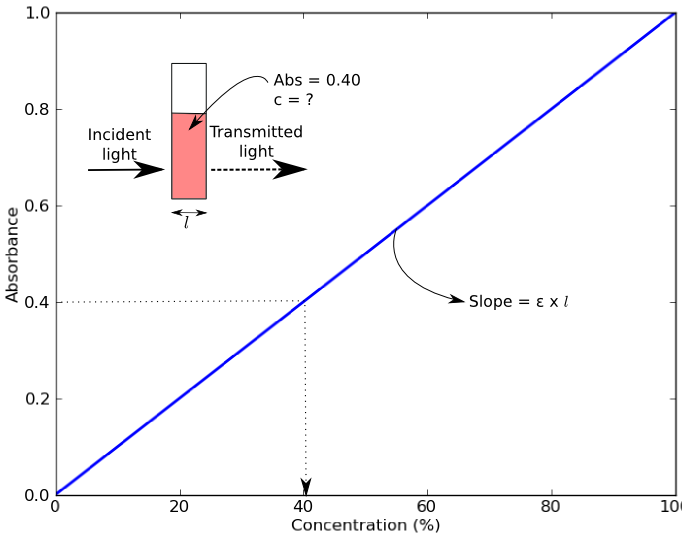
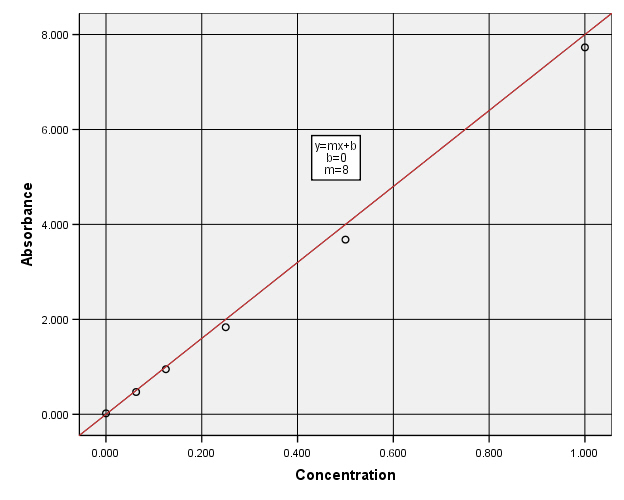
Use the data to plot a graph of Absorbance versus Concentration. Determine the concentration of the dye which would correspond to an absorbance of 0.140. What does Beer's Law say about the