Calculate the activation energy, E a Ea , in kilojoules per mole for a reaction at 57.0 ∘ C 57.0 ∘C that - brainly.com

Numerical on Arrhenius equation > The rate constant a first order reaction becomes six times when the temperature is raised from 350 to 4ook. Calculate Ea = ? Ang kesh R= 3:314J

The activation energy the reaction, 2 HI(g) + H2 + 12(g) is 209.5kJmol-1 581 K. Calculate the fraction of molecules of reactants having energy equal to or greater than activation energy? SOLUTION

Welcome to Chem Zipper.com......: In Arrhenius equation for a certain reaction, the value of A and Ea (activation energy) are 4 x10^13 sec^1 and 98.6 kJ mol1 respectively. At what temperature, the

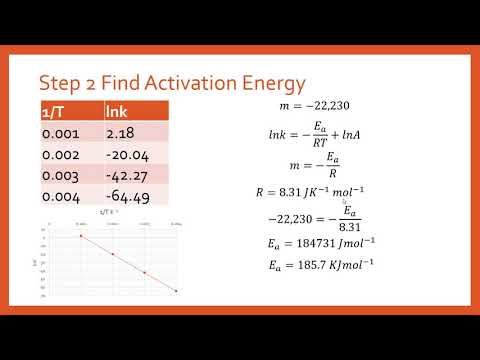
16.3.2 Determine activation energy (Ea) values from the Arrhenius equation by a graphical method. - YouTube

Welcome to Chem Zipper.com......: The rate of a reaction triple when temperature changes from 20”C to 50”C. Calculate energy of activation for the reaction (R = 8.314 JK^-1 mol^-1).
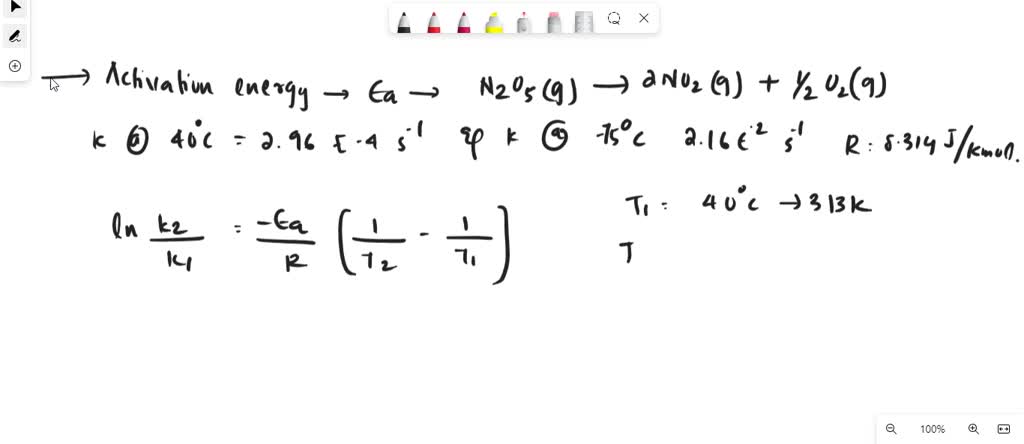
SOLVED: Calculate the activation energy, Ea, for N2O5(g) â†' 2 NO2(g) + 1/2 O2(g) given k (at 45.0 °C) = 5.79 × 10^-4 s^-1 and k (at 60.0 °C) = 3.83 ×

Calculation of activation energy (Ea) for the dose function. RH = 85%. | Download Scientific Diagram


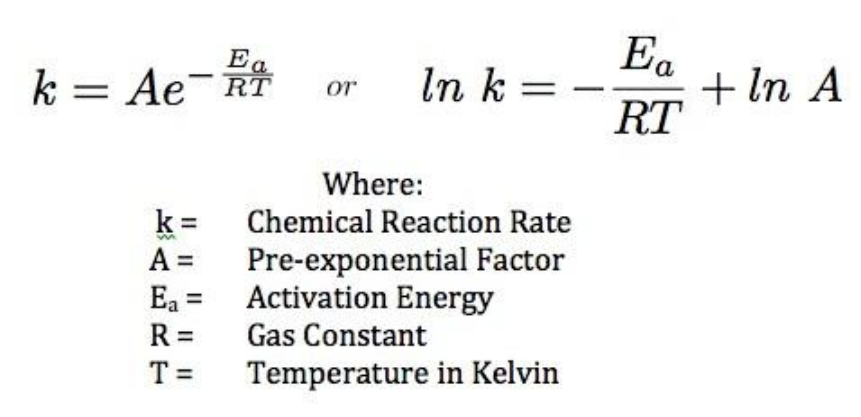

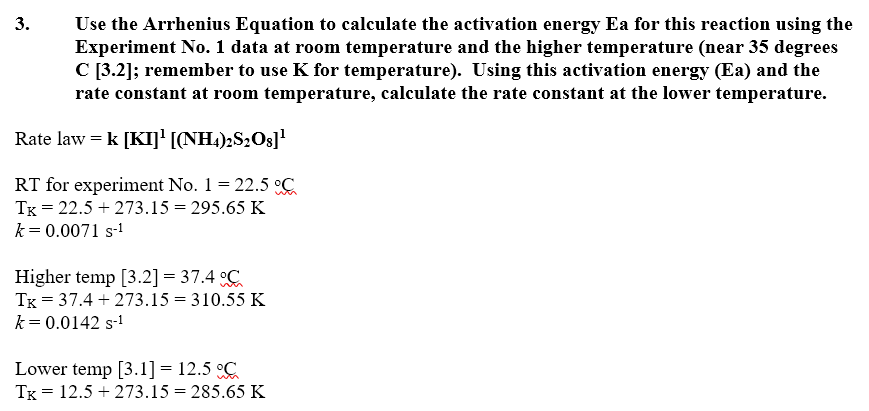
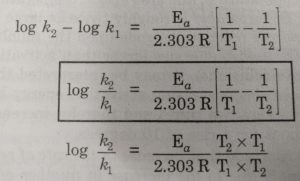
![Kannada] The rate constant of a reaction is doubled when the temperat Kannada] The rate constant of a reaction is doubled when the temperat](https://static.doubtnut.com/ss/web-overlay-thumb/6625533.webp)